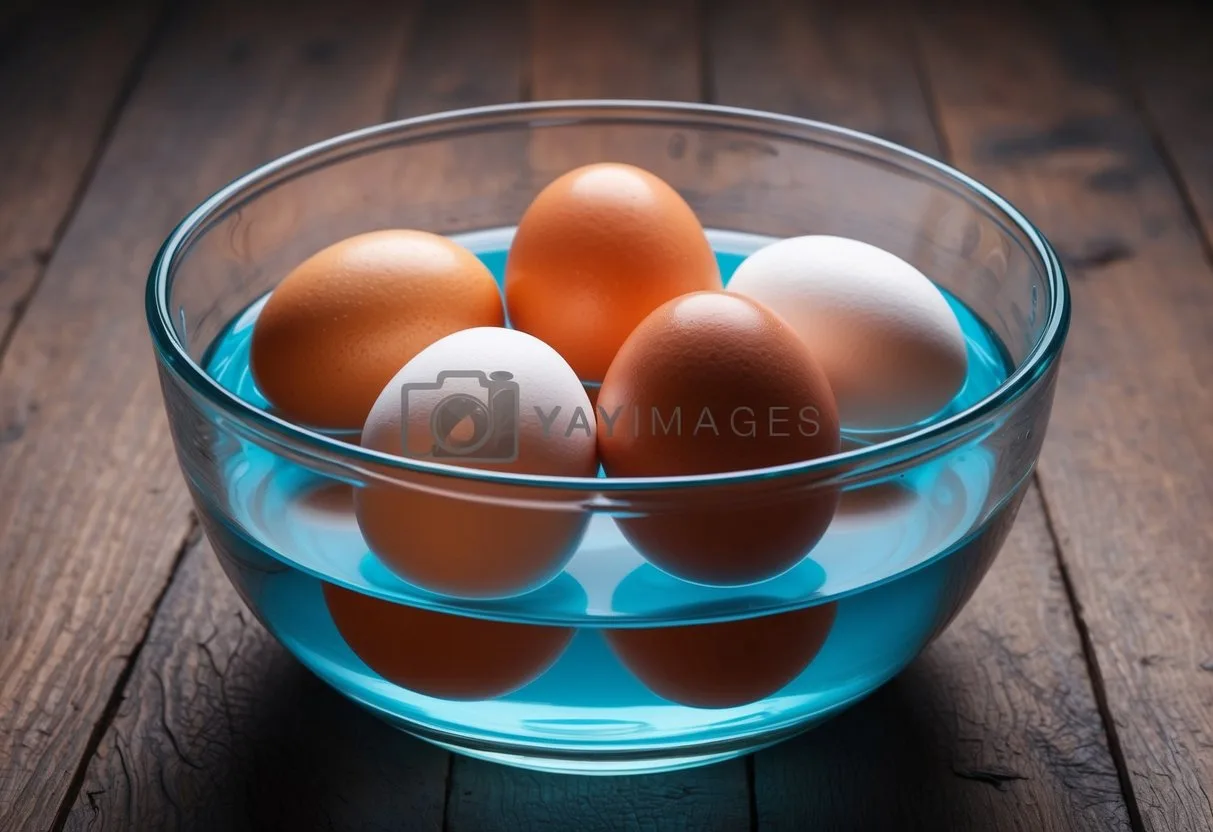
Have you ever wondered if those eggs in your fridge are still good to eat? The egg float test can help you find out. This simple method uses water to check if eggs are fresh or past their prime.
To do the test, put an egg in a bowl of cold water. If the egg sinks and lies flat, it’s fresh and safe to eat. If it stands up but still touches the bottom, it’s okay but should be used soon. An egg that floats to the top is likely spoiled and should not be eaten.
The float test works because of air pockets inside eggs. As eggs get older, these air pockets grow larger. This makes old eggs more likely to float in water. While not perfect, this test can be a handy way to check your eggs before cooking.
Understanding Eggs and Flotation
Eggs can float or sink in water depending on their age and condition. This neat trick helps us figure out if an egg is still good to eat. Let’s explore the science behind why some eggs float and others don’t.
The Science Behind Flotation
Eggs have a small air pocket inside them. As eggs get older, this air pocket grows bigger. A bigger air pocket makes the egg less dense. When an egg is less dense than water, it floats.
Fresh eggs are heavy and sink in water. They have small air pockets. As time passes, the egg loses moisture through its shell. This makes the air pocket bigger.
You can do a simple test at home. Put an egg in a bowl of water. If it sinks and lies flat, it’s very fresh. If it stands up but still touches the bottom, it’s okay to eat. If it floats, it’s best not to use it.
Specific Gravity and Buoyancy
Specific gravity is how dense something is compared to water. Eggs usually have a higher specific gravity than water, so they sink. But as they age, their specific gravity decreases.
Buoyancy is the force that makes things float. It depends on how much water the egg pushes aside. A fresh egg pushes aside less water than its weight, so it sinks. An old egg pushes aside more water than its weight, so it floats.
The density of the liquid matters too. In salty water, even fresh eggs might float because the water is denser. This is why the egg float test works best in plain water.
Exploring Salinity’s Role in Flotation
Salt changes how things float in water. It makes water denser, which affects what sinks or swims. Let’s look at how salt impacts water and how to make very salty water.
The Effect of Salt on Water Density
Salt makes water denser. When you add salt to water, it fills spaces between water molecules. This makes the water heavier for its size.
Saltier water can hold up more things. That’s why it’s easier to float in the ocean than in a lake. The Dead Sea is so salty that people can easily float on top of it.
You can see this effect with eggs. A fresh egg sinks in plain water. But add enough salt, and the egg will float! This happens because the salty water becomes denser than the egg.
Creating a Saturated Salt Solution
A saturated salt solution has as much salt as water can hold. Here’s how to make one:
- Start with warm water
- Add salt and stir
- Keep adding salt until it stops dissolving
When salt stops dissolving, you know the water can’t hold any more. This is your saturated solution.
You can use this super salty water for fun experiments. Try floating different objects in it. Compare how they float in regular water versus salt water.
Remember, different types of salt may behave a bit differently. Table salt (sodium chloride) is most common for these experiments.
Preparation for Egg Floatation Experiment
Getting ready for an egg floatation experiment is easy and fun. With a few simple items and steps, you can explore how eggs behave in different solutions.
Materials Needed for the Experiment
To do this cool experiment, you’ll need:
- 2-3 fresh eggs
- Water
- Salt
- 2 clear containers (glass or plastic)
- Measuring cups and spoons
- Spoon for stirring
- Pen and paper for notes
Make sure your containers are big enough to hold the eggs and water comfortably. Clear containers help you see what’s happening better.
Step-by-Step Procedure
-
Fill both containers with equal amounts of water. Use about 2 cups each.
-
Add salt to one container. Mix in 1/4 cup of salt and stir until it dissolves.
-
Gently place an egg in the container with plain water. Watch what happens and write it down.
-
Now put an egg in the salt water. Notice any differences and jot them down.
-
Measure the volume change in each container after adding the egg. This helps figure out the egg’s density.
-
Try adding more salt to see if it changes how the egg floats.
Remember to wash your hands after handling raw eggs. They can have germs.
Observing Flotation Dynamics
Watching eggs float or sink is fun and teaches us about density. We can learn a lot by carefully looking at how eggs behave in water and salt water.
Taking Measurements and Making Observations
Start by putting a plain egg in water. It will likely sink to the bottom. Write down where the egg sits in the glass.
Next, add salt to the water bit by bit. Stir it well each time. Keep an eye on the egg as you do this.
The egg may start to rise a little. It might even float to the top! Note how much salt you added to make this happen.
You can measure the egg’s position with a ruler. Mark where the egg sits in the water on the outside of the glass.
Egg flotation experiments help us see how density affects objects in water.
Logging Results and Observations
Keep a notebook to write down what you see. Make a simple table with columns for:
- Amount of salt added
- Egg position in water
- Any other things you notice
Draw pictures of the egg at different stages. This can help show how it moves.
Note the time for each step. Does the egg move right away when you add salt? Or does it take a while?
Look closely at the egg. Are there any bubbles on it? Does it tilt or spin?
Writing down these details helps scientists understand egg development and flotation.
Factors Affecting Flotation

Egg flotation depends on several key factors. These include the temperature of the water and how old the egg is.
Temperature’s Influence
Water temperature plays a big role in egg flotation. As water warms up, it becomes less dense. This change in density affects how eggs float or sink.
In cold water, eggs tend to sink more easily. The chilly water is denser, making it harder for eggs to float. As the water warms to room temperature, eggs may start to float more readily.
Some fun experiments involve testing eggs in different water temperatures. Try putting eggs in ice water, then in warm water. You might see the same egg sink in one and float in the other!
Egg Age and Flotation
An egg’s age is super important for flotation. Fresh eggs usually sink in water. They’re denser than water, so they drop to the bottom of a glass or bowl.
As eggs get older, they lose moisture through their shells. This creates an air pocket inside. The bigger this air pocket gets, the more likely the egg is to float.
You can do an egg float test at home. Put an egg in fresh water. If it sinks and lies flat, it’s very fresh. If it stands on its pointy end, it’s a bit older. Floating eggs are the oldest.
This test is a fun and easy way to check your eggs. But remember, floating doesn’t always mean the egg is bad. It’s just older and has a bigger air pocket.
Interpreting Flotation Results

The egg float test gives clues about an egg’s age and freshness. How an egg behaves in water tells us a lot about what’s happening inside the shell.
Density and Egg Flotation Outcomes
When you do the egg float test, you’re checking the egg’s density. Fresh eggs are dense and sink. As eggs age, they lose moisture and get less dense. This makes them float.
A very fresh egg will lie flat on the bottom of the bowl. It’s heavy and dense.
If an egg stands up but stays at the bottom, it’s a bit older but still good to eat.
An egg that floats to the top is old. The air inside has grown, making it less dense than water.
Analyzing Flotation Patterns
Watch how the egg moves in the water. This tells you more about its age.
Fresh eggs sink quickly and don’t move much. Older eggs might wobble or tilt as they sink.
The angle of a sinking egg matters too. The more it tilts, the older it is.
Eggs that float at different levels show different ages. Higher floaters are older than lower ones.
Specific gravity is key in this test. It’s about how dense the egg is compared to water. As the egg loses water, its specific gravity changes.
Scientific Principles and Flotation
Eggs can float or sink in water due to density differences. Adding salt to water changes its density, affecting how objects float. These concepts help teach important science lessons.
Charles E. Ophardt on Flotation
Charles E. Ophardt from Elmhurst College developed an egg flotation activity to teach density and buoyancy. Students add salt to fresh water until an egg floats. This hands-on experiment lets kids see density changes in action.
The activity teaches how to measure variables and record results. As salt dissolves, it increases the water’s density. When the water gets denser than the egg, the egg floats.
This simple setup uses common items like eggs, salt, and water. It’s a fun way for students to learn about density without complex equipment.
Educational Resources from Science Buddies
Science Buddies offers a similar egg floating activity with clear steps for kids. They suggest using 1 1⁄2 cups of water and 1⁄2 cup of salt to start.
Students add more water and stir to dissolve the salt. This gradual process helps them understand how salt concentration affects flotation.
The activity includes safety tips, like washing hands after handling raw eggs. It also explains why eggs float in saltwater but sink in fresh water. This helps kids connect the experiment to real-world situations.
Science Buddies provides extra questions to spark curiosity. These encourage students to think deeper about density and buoyancy principles.
Egg Flotation in Veterinary Science
Egg flotation is a key method vets use to find parasites in animals. It helps detect eggs and cysts in poop samples. Different solutions can make various parasite eggs float for easy viewing.
Fecal Flotation for Parasite Detection
Vets often use fecal flotation to find parasites in pets. This test mixes poop with a special liquid. Parasite eggs float to the top because they’re lighter than the liquid.
The vet puts some of the top layer on a slide. Then they look at it under a microscope. They can spot different types of parasite eggs this way.
Fecal flotation is great for finding worm eggs and some protozoa. It’s quick, cheap, and doesn’t hurt the animal. Vets can do it right in their office.
Different Flotation Solutions and Their Uses
Vets pick flotation solutions based on what parasites they want to find. Each solution has a different specific gravity. This affects which eggs will float.
Some common solutions are:
- Salt solution: Good for most roundworm and hookworm eggs
- Sugar solution: Helps float heavier eggs like tapeworm eggs
- Zinc sulfate: Great for finding protozoa cysts
The right solution is key. It can make the difference between seeing a parasite or missing it. Vets often use more than one type to catch different parasites.
Some eggs are too heavy to float in regular solutions. For these, vets might use special techniques or send samples to a lab.
Advanced Techniques in Egg Flotation
Egg flotation methods have improved over time. New tools and techniques allow for more precise measurements and better results.
Using a Hydrometer
A hydrometer helps measure the specific gravity of flotation solutions. This tool looks like a glass tube with a weighted bottom. You place it in the liquid and see where it floats.
For egg flotation, you want a solution with the right density. A hydrometer lets you check if it’s correct. This is key for getting good results.
To use a hydrometer:
- Fill a tall container with your flotation liquid
- Gently lower the hydrometer into it
- Let it settle and stop moving
- Read the number at the liquid’s surface
The reading tells you if your solution is too thick or thin. You can then adjust it as needed.
Sensitivity of Flotation Methods
Flotation methods can find even small numbers of parasite eggs. This makes them very useful for checking animal health.
Different methods have different levels of sensitivity. Some can detect as few as 1-2 eggs per gram of feces. Others need more eggs to work well.
Factors that affect sensitivity:
- Type of flotation solution used
- How the sample is prepared
- Time allowed for eggs to float
- Method of examining the sample
More sensitive methods take more time and skill. But they can catch problems earlier. This helps vets treat animals before they get very sick.
For the best results, labs often use a mix of techniques. This helps them catch different types of eggs and get a full picture of an animal’s health.
Flotation and Food Safety
Egg flotation can tell us about freshness, but it’s not the whole story when it comes to food safety. There are important things to know about eggs and harmful bacteria.
Link Between Egg Flotation and Salmonella
Egg flotation tests don’t show if eggs have Salmonella. A sinking egg can still have bacteria. Salmonella can grow inside eggs, even if the shell looks clean and unbroken.
Fresh eggs can have Salmonella too. The bacteria can be in the egg before the shell forms. This means even farm-fresh eggs need proper handling.
Cooking eggs well is key to killing Salmonella. The USDA says to cook eggs until both yolk and white are firm. This helps keep you safe from foodborne illness.
Storing Eggs: Best Practices and Myths
Proper egg storage is crucial for safety. You should keep eggs in the fridge at 40°F (4°C) or below. The cold slows bacteria growth.
Don’t store eggs at room temp. Some think this is okay, but it’s not safe. Warm air can make eggs sweat, helping bacteria spread.
The egg carton matters too. Keep eggs in their box in the main part of the fridge. Don’t use the door – it’s too warm there.
Check egg dates, but don’t rely on them alone. The float test can help, but it’s not perfect. When in doubt, throw it out.